@ WFS,World Fossil Society,Riffin T Sajeev,Russel T Sajeev
Paleontologists at the University of Toronto (U of T) and the Royal Ontario Museum (ROM) in Toronto have entirely revisited a tiny yet exceptionally fierce ancient sea creature called Habelia optata that has confounded scientists since it was first discovered more than a century ago.
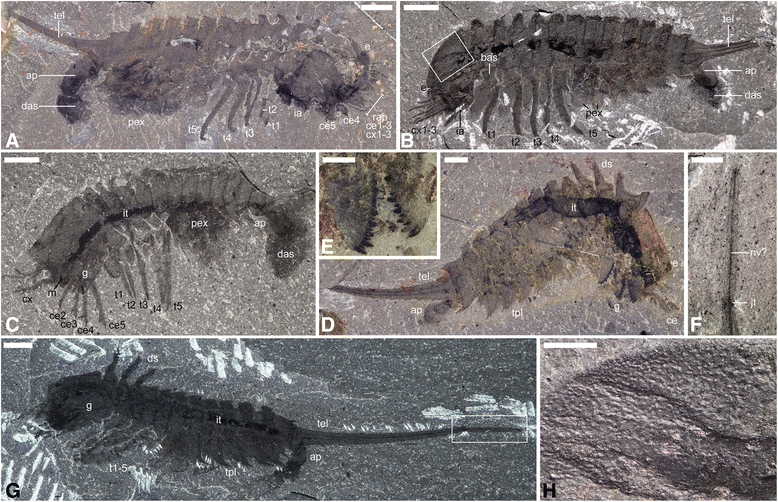
General anatomy of Habelia optata, morphs A (d-g) and B (a-c, h). a ROMIP 64357. b USNM 139209 (inset is (h)). c ROMIP 64358. d ROMIP 64359. e Close-up of the mandibles on the counterpart of (d) (wet specimen). f Close-up of the distal telson piece in (g) (wet specimen). g Holotype USNM 57693 (inset is (f)). h Close-up on cephalic ornamentation akin to trilobite prosopon in (b). All pictures taken under cross-polarized light. For abbreviations, see Methods. Scale bars: (a), 4 mm; (b), 3 mm; (c, d, g), 2 mm; (e, f, h), 1 mm
The research by lead author Cédric Aria, recent graduate of the PhD program in the department of ecology & evolutionary biology in the Faculty of Arts & Science at U of T, and co-author Jean-Bernard Caron, senior curator of invertebrate palaeontology at the ROM and an associate professor in the departments of ecology & evolutionary biology and Earth sciences at U of T, is published today in BMC Evolutionary Biology.
Approximately 2 cm in length with a tail as long as the rest of its body, the long-extinct Habelia optata belongs to the group of invertebrate animals called arthropods, which also includes such familiar creatures as spiders, insects, lobsters and crabs. It lived during the middle Cambrian period approximately 508 million years ago and comes from the renowned Burgess Shale fossil deposit in British Columbia. Habelia optata was part of the “Cambrian explosion,” a period of rapid evolutionary change when most major animal groups first emerged in the fossil record.

Anatomical and morphological details of Habelia optata, morphs A (b, d, f, n) and B (a, e, g, i, l, m). a USNM 139209, close-up of anterior cephalic area, showing intermediary appendage. b USNM 268931, cephalon, showing superimposed insertion of endopods on gnathobases; star points to insertion of anterior endopods. c ROMIP 64357, close-up of fourth cephalic exopodial branch, distal portion showing slender podomeres; arrow points to trident of setae at podomere junction. d ROMIP 64358, close-up of anteriormost region, showing mouth opening and first anterior pairs of gnathobases. e ROMIP 64360, close-up of teeth on masticatory margin of gnathobase; note heavy concentration of carbon in teeth. f Close-up of teeth on masticatory margin of posterior gnathobase on same specimen as in D, showing stronger carbon content in dental edge. g ROMIP 64364, specimen preserved in ventral aspect, close-up of anterior region showing labrum, eyes and appendages; star marks attachment of fifth spinose endopod; arrow points at ornamental spine of cephalic pleura; insets as indicated. h ROMIP 64362, close-up of posterior trunk exopods. i ROMIP 64363, close-up of anterior right cephalic region, dorsal view showing labrum and appendages; arrows point to overprint of gnathobases underneath cephalon. j, k ROMIP 64364. j Close-up of distal portion of cephalic endopod, showing “platform” with setal brushes. k Close-up of terminal claw; arrows point to teeth on inner margin of claw. l USNM 144907, close-up of cephalic gnathobases; arrows point to dentate margins of opposing gnathobases. m ROMIP 64357, close-up on anterior left cephalic region, showing appendages; arrow points to anterior insertion of fourth cephalic endopod. n ROMIP 64359, close-up of cephalic appendages showing insertion of endopods on gnathobases; star marks attachment of fourth cephalic endopod on its gnathobase. c-f, j and k are SEM images; all other are stereomicroscope images of dry specimens under cross-polarized lighting. For abbreviations, see Methods. Scale bars: (a, g, h, i, l, n), 1 mm; (b, m), 0.5 mm; (c, d, k), 200 μm; (e), 100 μm; (f), 50 μm; (j), 500 μm
Like all arthropods, Habelia optata features a segmented body with external skeleton and jointed limbs. What remained unclear for decades, however, was the main sub-group of arthropods to which Habelia belonged. Early studies had mentioned mandibulates — a hyperdiverse lineage whose members possess antennae and a pair of specialized appendages known as mandibles, usually used to grasp, squeeze and crush their food. But Habelia was later left as one of the typically unresolved arthropods of the Burgess Shale.
The new analysis by the U of T-ROM researchers suggests that Habelia optata was instead a close relative of the ancestor of all chelicerates, the other sub-group of arthropods living today, named for the presence of appendages called chelicerae in front of the mouth and used to cut food. This is mostly due to the overall anatomy of the head in Habelia, and the presence of two small chelicerae-like appendages revealed in these fossils.
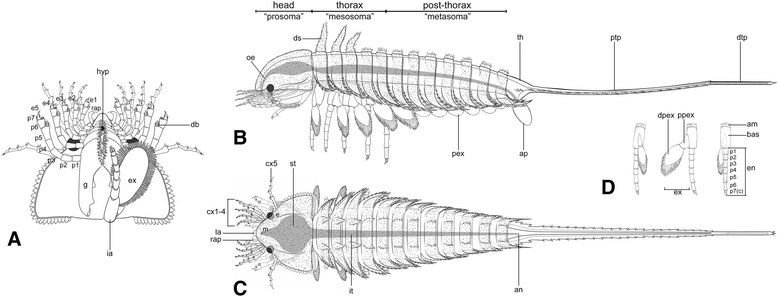
Diagrammatic reconstruction of Habelia optata, morph A. a Ventral view of the cephalon. Right “intermediary” appendage removed to show gnathobase morphology. b Lateral view. c Dorsal view. d Isolated biramous thoracic limb in frontal, lateral and posterior views (left to right). For abbreviations, see Methods. Line drawings courtesy of Joanna Liang © Royal Ontario Museum
“Habelia now shows in great detail the body architecture from which chelicerates emerged, which allows us to solve some long-standing questions,” said Aria, who is now a post-doctoral researcher at the Nanjing Institute of Geology and Palaeontology, in China. “We can now explain why, for instance, horseshoe crabs have a reduced pair of limbs — the chilaria — at the back of their heads. Those are relics of fully-formed appendages, as chelicerates seem to originally have had heads with no less than seven pairs of limbs.”
Aria and Caron analyzed 41 specimens in total, the majority of which are new specimens acquired by ROM-led fieldwork parties to the Burgess Shale.
The research illustrates that the well-armoured body of Habelia optata, covered in a multitude of different spines, was divided into head, thorax and post-thorax, all bearing different types of appendages. The thorax displays five pairs of walking legs, while the post-thorax houses rounded appendages likely used in respiration.
“Scorpions and the now-extinct sea scorpions are also chelicerates with bodies divided into three distinct regions,” Aria explained. “We think that these regions broadly correspond to those of Habelia. But a major difference is that scorpions and sea scorpions, like all chelicerates, literally ‘walk on their heads,’ while Habelia still had walking appendages in its thorax.”
The researchers argue that this difference in anatomy allowed Habelia to evolve an especially complex head that makes this fossil species even more peculiar compared to known chelicerates. The head of Habelia contained a series of five appendages made of a large plate with teeth for mastication, a leg-like branch with stiff bristle-like spines for grasping, and an elongate, slender branch modified as a sensory or tactile appendage.
“This complex apparatus of appendages and jaws made Habelia an exceptionally fierce predator for its size,” said Aria. “It was likely both very mobile and efficient in tearing apart its preys.”
The surprising outcome of this study, despite the evolutionary relationship of Habelia with chelicerates, is that these unusual characteristics led instead the researchers to compare the head of Habelia with that of mandibulates from a functional perspective. Thus, the peculiar sensory branches may have been used in a similar fashion as mandibulates use antennae. Also, the overlapping plate-like appendages in the middle series of five are shown to open and close parallel to the underside of the head — much as they do in mandibulates, especially those that feed on animals with hardened carapaces.
Lastly, a seventh pair of appendages at the back of the head seems to have fulfilled a function similar to that of “maxillipeds” — appendages in mandibulates that assist with the other head limbs in the processing of food. This broad correspondence in function rather than in evolutionary origin is called “convergence.”
“From an evolutionary point of view, Habelia is close to the point of divergence between chelicerates and mandibulates,” Aria said. “But its similarities with mandibulates are secondary modifications of features that were in part already chelicerate in nature. This suggests that chelicerates originated from species with a high structural variability.”
The researchers conclude from the outstanding head structure, as well as from well-developed walking legs, that Habelia optata and its relatives were active predators of the Cambrian sea floors, hunting for small shelly sea creatures, such as small trilobites — arthropods with hard, mineralized exoskeletons that were already very diverse and abundant during Cambrian times.
“This builds onto the importance of carapaces and shells for evolutionary change during the Cambrian explosion, and expands our understanding of ecosystems at this time, showing another level of predator-prey relationship and its determining impact on the rise of arthropods as we know them today,” said Caron, who was Aria’s PhD supervisor when the bulk of this research was completed.
“The appearance and spread of animals with shells are considered to be one of the defining characteristics of the Cambrian explosion, and Habelia contributes to illustrate how important this ecological factor was for the early diversification of chelicerates and arthropods in general.”
- Cédric Aria, Jean-Bernard Caron. Mandibulate convergence in an armoured Cambrian stem chelicerate. BMC Evolutionary Biology, 2017; 17 (1) DOI: 10.1186/s12862-017-1088-7
- University of Toronto. “A 508-million-year-old sea predator with a ‘jackknife’ head: Oldest close parent of spiders, scorpions and horseshoe crabs evolved sophisticated head to hunt and eat small shelly animals.” ScienceDaily. ScienceDaily, 22 December 2017. <www.sciencedaily.com/releases/2017/12/171222090334.htm>.
@ WFS,World Fossil Society,Riffin T Sajeev,Russel T Sajeev



 December 23rd, 2017
December 23rd, 2017  Riffin
Riffin 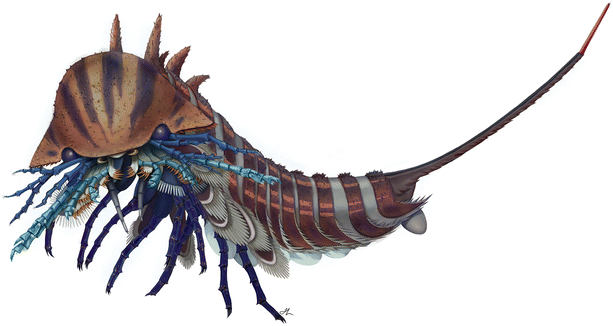
 Posted in
Posted in  Tags:
Tags: 