@ WFS,World Fossil Society,Riffin T Sajeev,Russel T Sajeev
Flowering plants likely originated between 149 and 256 million years ago according to new UCL-led research.
The study, published today in New Phytologist by researchers from the UK and China, shows that flowering plants are neither as old as suggested by previous molecular studies, nor as young as a literal interpretation of their fossil record.
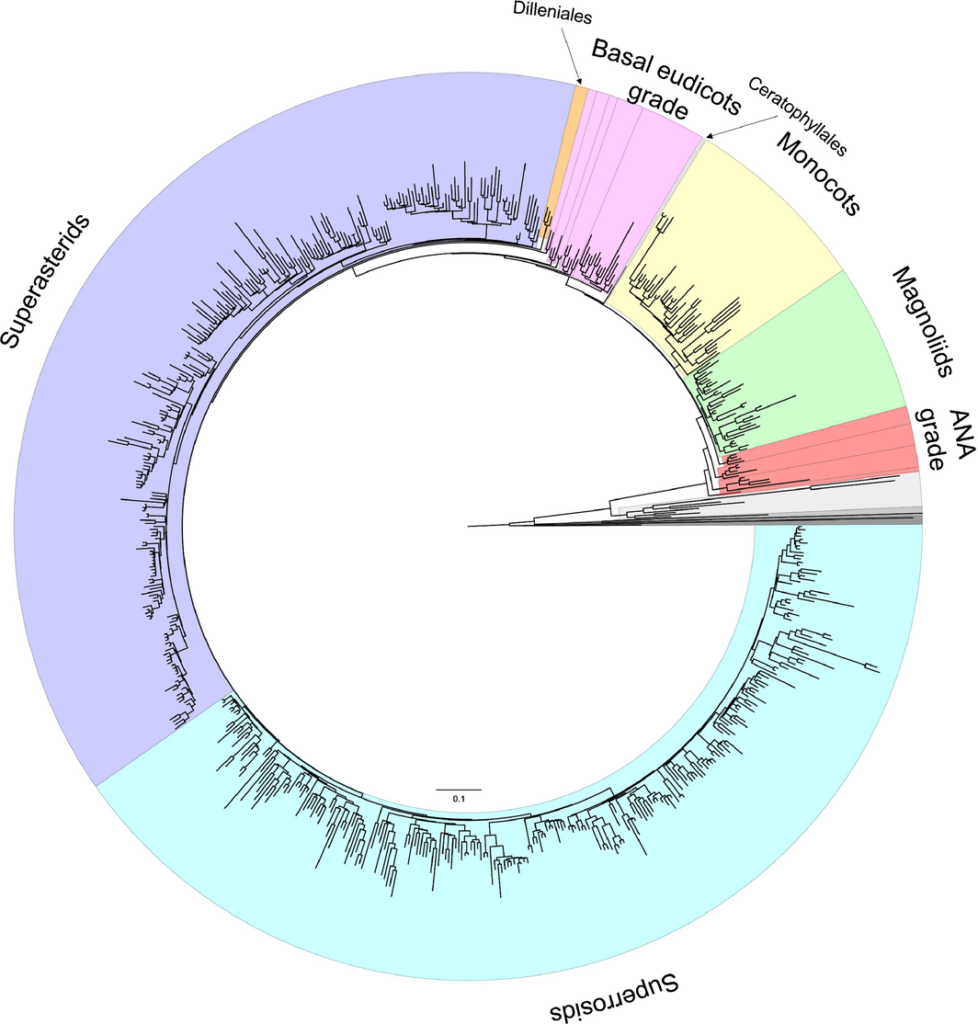
RAxML tree estimated from the 83 genes and 644 taxa of tracheophytes. The major angiosperm lineages and grades are highlighted: ANA grade (red), magnoliids (green), monocots (yellow), Ceratophyllales (pale blue), basal eudicots grade (pink), Dilleniales (orange), superasterids (purple) and superrosids (blue). Species names and bootstrap support values are indicated in Supporting Information Fig. S3
The findings underline the power of using complementary studies based on molecular data and the fossil record, along with different approaches to infer evolutionary timescales to establish a deeper understanding of evolutionary dynamics many millions of years ago.
“The discrepancy between estimates of flowering plant evolution from molecular data and fossil records has caused much debate. Even Darwin described the origin of this group as an ‘abominable mystery’,” explained lead author, Dr Jose Barba-Montoya (UCL Genetics, Evolution & Environment).
![Summary tree of tracheophytes showing fossil calibrations. Calibrations are represented for 52 nodes, consisting of (>) soft minimum (closed red dots) or both ([min, max]) soft minimum and soft maximum (open red dots). Calibrated nodes are numbered as in Supporting Information Fig. S2. Justifications for these minima and maxima are provided in Notes S1 and an overview in Table S3. The dagger symbol shows a species that is extinct. The tree has been scaled to time on the basis of the minimum constraints.](http://www.worldfossilsociety.org/wp-content/uploads/2018/02/02-pterosaur-eggs-wang6HR.adapt_.768.1-1-805x1024.png)
Summary tree of tracheophytes showing fossil calibrations. Calibrations are represented for 52 nodes, consisting of (>) soft minimum (closed red dots) or both ([min, max]) soft minimum and soft maximum (open red dots). Calibrated nodes are numbered as in Supporting Information Fig. S2. Justifications for these minima and maxima are provided in Notes S1 and an overview in Table S3. The dagger symbol shows a species that is extinct. The tree has been scaled to time on the basis of the minimum constraints.
Molecular-clock dating studies, however, have suggested a much older origin for flowering plants, implying a cryptic evolution of flowers that is not documented in the fossil record.
“In large part, the discrepancy between these two approaches is an artefact of false precision on both palaeontological and molecular evolutionary timescales,” said Professor Philip Donoghue from the University of Bristol’s School of Earth Science, and a senior author of the study.
Palaeontological timescales calibrate the family tree of plants to geological time based on the oldest fossil evidence for its component branches. Molecular timescales build on this approach, using additional evidence from genomes for the genetic distances between species, aiming to overcome gaps in the fossil record.
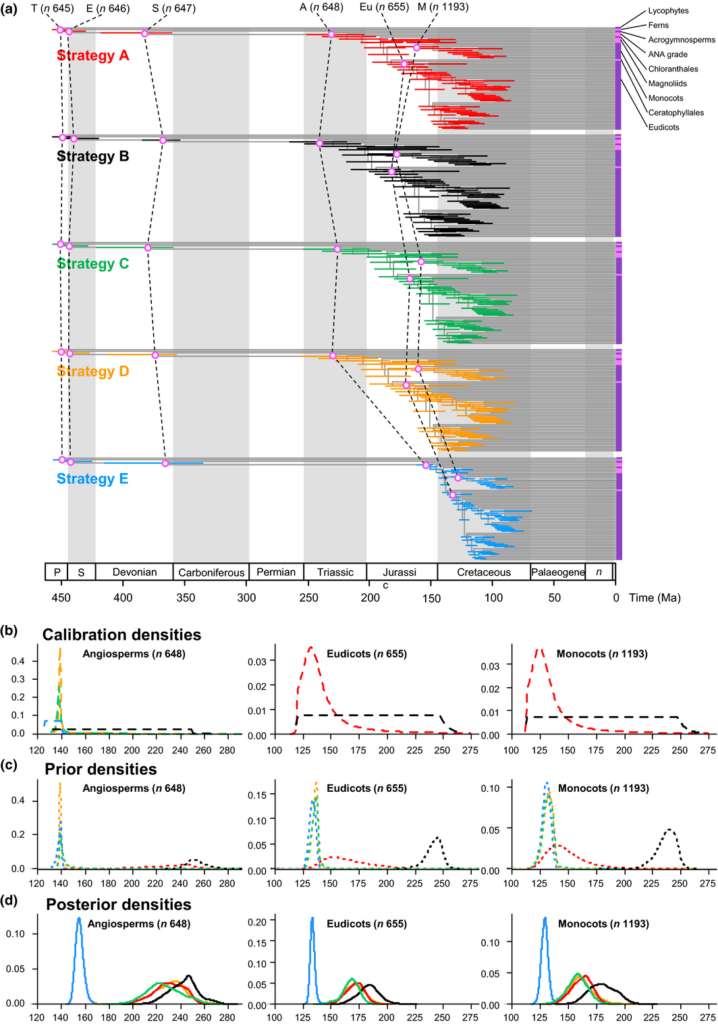
The effect of calibrations on posterior divergence time estimates of major groups of tracheophytes and angiosperms. (a) Summary chronogram for tracheophytes (including two lycophytes, two ferns, eight gymnosperms and 64 orders of angiosperms) with terminals collapsed to represent angiosperm orders showing divergence time estimates. Nodes are drawn at the posterior means obtained and horizontal bars represent 95% high posterior density (HPD) credibility intervals (CIs). Estimates were obtained using the HKY85 + Γ5 substitution model, independent rates model (IR), with the 83 genes subdivided into three partitions: 1st and 2nd codon positions for plastid genes; 1st and 2nd codon positions for mitochondrial genes; and nuclear RNA genes. Five nodes are connected (purple open dots) across the analyses to facilitate comparison: tracheophytes (n = 645), seed plants (n = 647), angiosperms (n = 648), eudicots (n = 655) and monocots (n = 1193). (b–d) Calibration, prior and posterior densities for three angiosperm nodes in the tracheophyte phylogeny. Colouring relates to the calibration strategy as in (a). The phylogeny with clade names is provided in Fig. 6. Nodes in parentheses are numbered as in Supporting Information Fig. S2.
“Previous studies into molecular timescales failed to explore the implications of experimental variables and so they inaccurately estimate the probable age of flowering plants with undue precision,” said Professor Ziheng Yang (UCL Genetics, Evolution & Environment) and senior author of the study.
“Similarly, interpretations of the fossil record have not fully recognised its shortcomings as an archive of evolutionary history, that is, that the oldest fossil evidence of flowering plants comes from very advanced, not primitive flowering plant lineages,” Professor Donoghue added.
The researchers compiled a large collection of genetic data for many flowering plant groups including a dataset of 83 genes from 644 taxa, together with a comprehensive set of fossil evidence to address the timescale of flowering plant diversification.
“By using Bayesian statistical methods that borrow tools from physics and mathematics to model how the evolutionary rate changes with time, we showed that there are broad uncertainties in the estimates of flowering plant age, all compatible with early to mid-Cretaceous origin for the group,” said Dr Mario dos Reis (School of Biological and Chemical Sciences at Queen Mary University of London), a co-author of the study.
- Jose Barba-Montoya, Mario dos Reis, Harald Schneider, Philip C. J. Donoghue, Ziheng Yang. Constraining uncertainty in the timescale of angiosperm evolution and the veracity of a Cretaceous Terrestrial Revolution. New Phytologist, 2018; DOI: 10.1111/nph.15011



 February 11th, 2018
February 11th, 2018  Riffin
Riffin  Posted in
Posted in  Tags:
Tags: 