@WFS,World Fosssil Society,Riffin T Sajeev,Russel T Sajeev
Spherical, isolated carbonate concretions occur throughout the world in marine argillaceous sedimentary rocks of widely varying geological ages. These concretions are characteristically highly enriched in CaCO3compared to the surrounding sedimentary rock matrices and are typically separated from these rock matrices by sharp boundaries1,2,3. These sharp boundaries mean that the isolated carbonate concretions are readily identifiable and both geologists and non-scientists alike have been motivated to consider how such concretions could have formed.

Conceptual view of the features of spherical concretions. Ca profile and porosity distribution across the spherical concretion forming under rather stable conditions under which solutes diffused continuously, causing calcite precipitation until the organic source of carbon at the centre of the concretion was consumed.
It is also known that many of these concretions have various kinds of well-preserved fossils at their centres3. Isotopic analyses have been used to identify the source of carbon that formed the concretions4,5,6and to understand the diagenetic processes, including microbial activity, that occurred during sediment burial and concretion formation1,7,8. However, even though there have been many studies of concretions over several decades, there still remain questions regarding concretion formation9,10,11. The remaining questions include: How rapidly do concretions grow? Why are the concretions often spherical with sharp boundaries? and Why does the localization of Ca and CO3, and consequently concretion growth, stop? These questions can be answered by determining the relationship between the formation conditions of the spherical concretions, the mass transport processes in the sedimentary matrix, and the concretion growth rates. However, this relationship has yet to be described precisely.
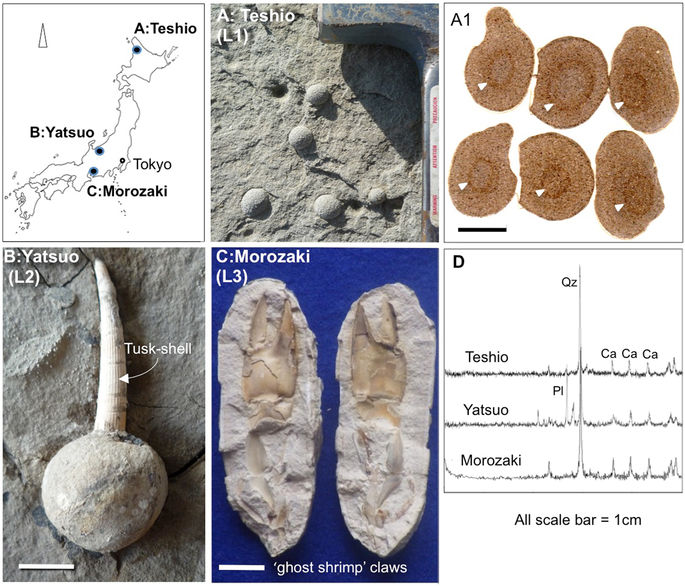
Sampling locations and the characteristics in hand specimen of studied Japanese spherical carbonate concretions. (A) Teshio concretions (L1); (B) Yatsuo tusk-shell concretions (L2), and (C) Morozaki ‘ghost-shrimp’ concretions (L3). Teshio concretions from Hokkaido with rounded impressions in their centers suggesting the presence of a non-skeletal animal (arrows: A1). δ13C data also suggest an organic carbon origin. (D) X-ray diffractograms for each concretion shows a clear calcite peak. Index map is based on the data of Geospatial Information Authority of Japan website (http://www.gsi.go.jp/maps.gsi.go.jp). All photographs (A–C) shown here are taken by H.Yoshida.
For several decades, the formation of spherical concretions has been explained by diffusion accompanied by carbonate inter-conversion reactions12,13 and also by very slow three dimensional advection of water when cementation conditions are isotropic9,14. These processes give rise to a spherical morphology in homogeneous sediments or sedimentary rocks. The process is basically accepted, but previously proposed models cannot explain the steep chemical gradients (notably of Ca) that occur across the margins of almost all concretions, and also the constant calcite (CaCO3) concentration3,15 and constant porosity within the body of a concretion16,17, as determined by thin section analysis and porosity measurements3. Such a steep boundary occurs as the reaction front on the concretion surface (Fig. 1) is developed by reactions between HCO3− and Ca2+ ions as concretions grow outwards, and has a certain characteristic width ‘L’ where CaCO3 has been precipitated. This front is developed in any kind of carbonate-rich spherical concretion formed syn-genetically during burial of marine sediments with organic carbon sources in the concretions3,18,19. In this case, the relationship between ‘L’, diffusion coefficient ‘D’ of HCO3− and growth rate of the concretion ‘V’ is: ‘L = D/V’. This relationship can be illustrated using a ‘Diffusion-growth rate cross-plot’, which was developed to explain detailed observations on concretions at a single locality in Japan3. Here, we extend the theory of the ‘cross-plot diagram’ by applying it to concretions of different ages at several localities to develop a general model for the formation conditions of spherical carbonate concretions in marine sediments. The outcome of the analysis is also used to answer the above remaining questions about syn-genetically formed spherical carbonate concretions and to build on the previous study, which only investigated concretions from a single locality.
- Raiswell, R. The microbiological formation of carbonate concretions in the Upper Lias of NE England. Chemical Geology18, 227–244 (1976).
-
Dix, G. R. & Mullins, H. T. Shallow, Subsurface growth and burial alteration of Middle Devonian calcite concretions. Jour. Sedimentary Petology 57, 140–152 (1987).
-
Yoshida, H. et al. Early post-mortem formation of carbonate concretions around tusk-shells over week-month timescales. Scientific Reports 5, 14123, https://doi.org/10.1038/srep14123(2015).
-
Curtis, C. D., Coleman, M. L. & Love, L. G. Pore water evolution during sediment burial from isotopic and mineral chemistry of calcite, dolomite and siderite concretions. Geochim. Cosmochim. Acta 50, 2321–2334 (1986).
-
Mozley, P. & Burns, S. J. Oxygen and carbon isotopic composition of marine carbonate concretions: An overview. Jour. Sedim. Petrol.63, 73–83 (1993).
-
Raiswell, R. & Fisher, Q. J. Mudrock-hosted carbonate concretions. a review of growth mechanisms and their influence on chemical and isotopic composition. Jour. Geological Society, London 157, 239–251 (2000).
-
Irwin, H., Curtis, C. & Coleman, M. Isotopic evidence for source of diagenetic carbonates formed during burial of organic-rich sediments. Nature 269, 209–213 (1977).
-
Coleman, M. L. Microbial processes: Controls on the shape and composition of carbonate concretions. Marine Geology 113, 127–140 (1993).
-
Seilacher, A. Concretion morphologies reflecting diagenetic and epigenetic pathways. Sedimentary Geology 143, 41–57 (2001).
-
Wilson, D. D. & Brett, C. E. Concretions as sources of exceptional preservation, and decay as a source of concretions: examples from the Middle Devonian of New York. Palaios 28, 305–316 (2012).
-
Mccoy, V. E., Young, R. T. & Briggs, D. E. G. Sediment permeability and the preservation of soft-tissues in concretions: an experimental study. Palaios 30, 608–612 (2015).
-
Berner, R. A. Rate of concretion growth. Geochim. Cosmochim. Acta 32, 477–483 (1968a).
-
Wilkinson, M. & Damper, M. D. The rate of growth of sandstone-hosted calcite concretions. Geochim. Cosmochim. Acta 54, 3391–3399 (1990).
-
Mozley, P. & Davis, J. M. Internal structure and mode of growth of elongate calcite concretions: Evidence for small-scale, microbially induced, chemical heterogeneity in groundwater. GSA Bulletin 117, 1400–1412 (2005).
-
Coleman, M. L. & Raiswell, R. Carbon, oxygen and sulphur isotope variations in concretions from the Upper Lias of NE England. Geochim. Cosmochim. Acta 45, 329–340 (1981).
-
Raiswell, R. The growth of Cambrian and Liassic Concretions. Sedimentology 17, 147–171 (1971).
-
Curtis, D. C., Petrowski, C. & Oertel, G. Stable carbon isotope ratios within carbonate concretions: a clue to place and time of formation. Nature 235, 98–100 (1972).
-
Berner, R. A. Calcium carbonate concentrations formed by the decomposition of organic matter. Science 159, 195–197 (1968b).
-
Coleman, M. L. & Raiswell, R. Source of carbonate and origin of zonation in pyritiferous carbonate concretions: evaluation of a dynamic model. American Journal of Science 295, 282–308 (1995).
-
Takahashi, A., Hirano, H. & Sato, T. Stratigraphy and fossil assemblage of the Upper Cretaceous in the Teshionakagawa area, Hokkaido, northern Japan. Journal of Geological Society of Japan.109(2), 77–95 (2003).
-
Yanagisawa, Y. Diatom biostratigraphy of the lower to middle Miocene sequence in the Yatsuo area, Toyama Prefecture, central Japan. Bull. Geol. Surv. Japan 50, 139–165 (1999).
-
Itoh, Y., Yanagisawa, Y. & Watanabe, M. Magnetostratigraphy and diatom biostratigraphy of Neogene rocks distributed in the Yatsuo area, central Japan. Bull. Geol. Surv. Japan 50, 215–223 (1999).
-
Kondo, Y. & Kimura, I. Geology of the Morozaki district: Geological Sheet Map at 1: 50000, Geological Survey of Japan (in Japanese with English abstract) (1987).
-
Matumoto, T. Cretaceous Formation in Hokkaido area, Japan (No. 2). Journal of Geological Society of Japan, 296–297 (1939).
-
Nagao, T. On some fossil Crustacea from Japan. Journal of the Faculty of Science, Hokkaido Imperial University. 4, 85–100 (1941).
-
Hesselbo, S. P. & Palmer, T. J. Reworked early diagenetic concretions and the bioerosional origin of a regional discontinuity within British Jurassic marine mudstones. Sedimentology 39, 1045–1065 (1992).
-
Boles, J. R., Landis, C. A. & Dale, P. The Moeraki boulders – anatomy of some septarian concretions. Jour. Sedimentary Geology 55, 398–406 (1985).
-
Thyne, G. D. & Boles, J. R. Isotopic evidence for origin of the Moeraki septarian concretions, New Zealand. Jour. Sedimentary Geology 59, 272–279 (1989).
-
Li, Y. H. & Gregory, S. Diffusion of ions in sea-water and in deep-sea sediments. Geochim. Cosmochim. Acta 38, 703–714 (1974).
-
Lebron, I. & Surez, D. L. Calcite nucleation and precipitation kinetics as affected by dissolved organic matter at 25 °C and pH 7.5. Geochim. Cosmochim. Acta 60, 2765–2776 (1996).
-
Barnaby, R. J. & Rimstidt, J. D. Redox conditions of calcite cementation interpreted from Mn and Fe contents of authigenic calcites. Geol. Soc. Am. Bull. 101, 795–804 (1989).
-
Spaargaren, D. H. Hydrodynamic properties of benthic marine Crustacea. I. Specific gravity and drag coefficients. Mar. Ecol. Prog. Ser. 1, 351–359 (1979).
@WFS,World Fosssil Society,Riffin T Sajeev,Russel T Sajeev



 May 10th, 2018
May 10th, 2018  Riffin
Riffin  Posted in
Posted in  Tags:
Tags: 