For example if you have a fossil trilobite and it was found in the Wheeler Formation. The Wheeler Formation has been previously dated to approximately 507 million year old, so we know the trilobite is also about 507 million years old. But, how can we determine how old a rock formation is, if it hasn’t previously been dated?
Scientists can use certain types of fossils referred to as index fossils to assist in relative dating via correlation. Index fossils are fossils that are known to only occur within a very specific age range. Typically commonly occurring fossils that had a widespread geographic distribution such as brachiopods, trilobites, and ammonites work best as index fossils. If the fossil you are trying to date occurs alongside one of these index fossils, then the fossil you are dating must fall into the age range of the index fossil.
Sometimes multiple index fossils can be used. In a hypothetical example, a rock formation contains fossils of a type of brachiopod known to occur between 410 and 420 million years. The same rock formation also contains a type of trilobite that was known to live 415 to 425 million years ago. Since the rock formation contains both types of fossils the ago of the rock formation must be in the overlapping date range of 415 to 420 million years.
Studying the layers of rock or strata can also be useful. Layers of rock are deposited sequentially. If a layer of rock containing the fossil is higher up in the sequence that another layer, you know that layer must be younger in age. If it is lower in sequence it’s of a younger age. This can often be complicated by the fact that geological forces can cause faulting and tilting of rocks.
Absolute dating is used to determine a precise age of a rock or fossil through radiometric dating methods. This uses radioactive minerals that occur in rocks and fossils almost like a geological clock. It’s often much easier to date volcanic rocks than the fossils themselves or the sedimentary rocks they are found in. So, often layers of volcanic rocks above and below the layers containing fossils can be dated to provide a date range for the fossil containing rocks.
The atoms in some chemical elements have different forms, called isotopes. These isotopes break down at a constant rate over time through radioactive decay. By measuring the ratio of the amount of the original (parent) isotope to the amount of the (daughter) isotopes that it breaks down into an age can be determined.
We define the rate of this radioactive decay in half-lives. If a radioactive isotope is said to have a half-life of 5,000 years that means after 5,000 years exactly half of it will have decayed from the parent isotope into the daughter isotopes. Then after another 5,000 years half of the remaining parent isotope will have decayed.
While people are most familiar with carbon dating, carbon dating is rarely applicable to fossils. Carbon-14, the radioactive isotope of carbon used in carbon dating has a half-life of 5730 years, so it decays too fast. It can only be used to date fossils younger than about 75,000 years. Potassium-40 on the other hand has a half like of 1.25 billion years and is common in rocks and minerals. This makes it ideal for dating much older rocks and fossils.



 March 21st, 2018
March 21st, 2018  Riffin
Riffin 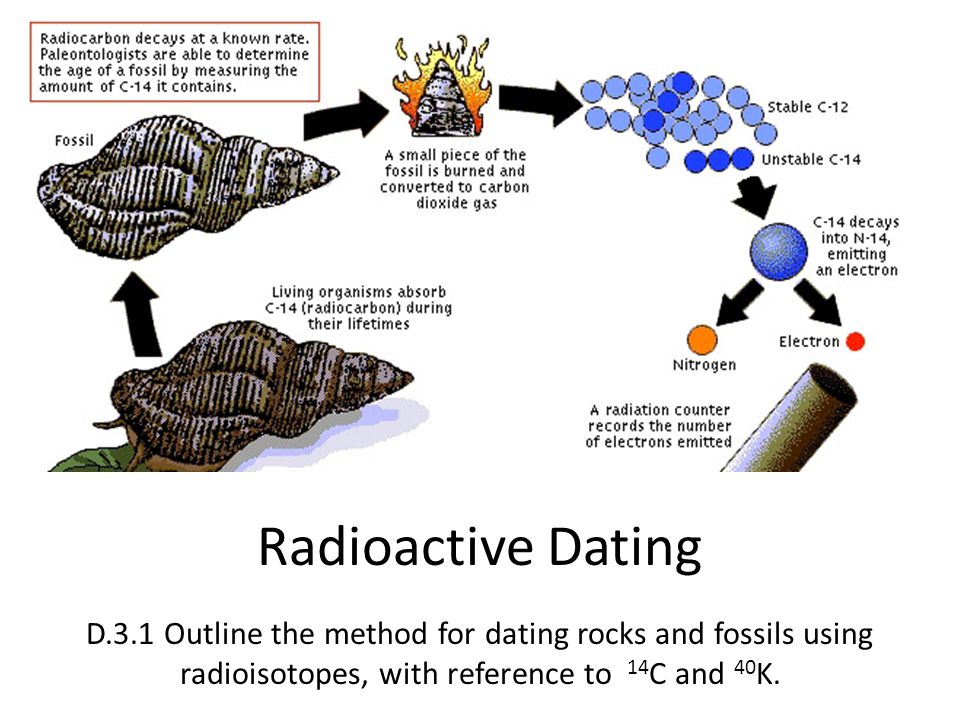
 Posted in
Posted in  Tags:
Tags: 