@WFS,World Fossil Society, Athira, Riffin T Sajeev, Russel T Sajeev

Bone cavities, known as air sacs, emerged in precursors of long-necked dinosaurs roughly 225 million years ago, as evidenced by a specimen unearthed in Rio Grande do Sul, South Brazil. The research also indicates that air sacs did not evolve as linearly as scientists believe. Credit: Márcio L. Castro
Bone cavities called air sacs emerged in the precursors of long-necked dinosaurs around 225 million years ago, according to the analysis of a specimen found in Rio Grande do Sul state, South Brazil.
The missing link has just been found, bridging the gap between the earliest dinosaurs, which varied significantly in size from mere centimeters to a maximum of 3 meters, and more recent giants that could be more than twice the length of a bus and have so much appeal to the popular imagination.
Macrocollum itaquii, which was discovered in the region of Agudo in the Rio Grande do Sul state of South Brazil and dates back 225 million years, is the most ancient dinosaur known to have structures referred to as air sacs.
These bone cavities, which persist in present-day birds, enabled dinosaurs to capture more oxygen, keep their bodies cool, and withstand the harsh conditions of their era. They also helped some become giants: Tyrannosaurus rex and Brachiosaurus, for example.
An article on the study that led to the discovery was published in the journal Anatomical Record. Two of its authors are researchers supported by FAPESP at the State University of Campinas (UNICAMP) in São Paulo state.
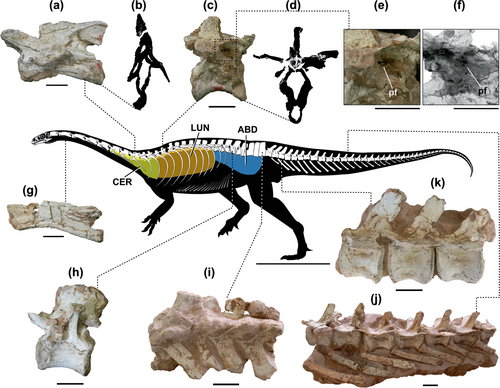
Skeletal reconstruction of the unaysaurid sauropodomorph Macrocollum (CAPPA/UFSM 0001b) showing vertebral elements along the spine and putative reconstruction of the air sac systems involved. (a) Pneumatic posterior cervical vertebra and a cross-section CT slice in b. (c) a pneumatized anterior dorsal vertebra with cross-section CT slice in d, and detail of the pneumatic foramen in e. (f) Detail of the pneumatic foramen in a reconstructed 3D model of the element. (g) Anterior cervical element (apneumatic). (h) Posterior dorsal vertebra shows no traces of PSP. The sacral series (i), as well as the anterior (k) and mid-caudal (j) series are apneumatic. a, g, h, j, and k are in left lateral view. c, e and f are in right lateral view. i is in dorsal view. ABD, abdominal diverticula; CER, cervical diverticula; LUN, lung; pf, pneumatic foramen. The reconstruction was made by Rodrigo T. Müller. Scale bar of the skeletal reconstruction = 500 mm; a–j = 20 mm.
“Air sacs made their bones less dense, allowing them to grow to more than 30 meters in length,” said Tito Aureliano, first author of the article. The study was conducted as part of his PhD research at the Institute of Geosciences (IG-UNICAMP).
“M. itaquii was the largest dinosaur of its time, with a length of about 3 meters. A few million years before then, the largest dinosaurs were about 1 meter long. Air sacs certainly facilitated this increase in size,” Aureliano added.
The study was a stage of the project “Taphonomic landscapes,” funded by FAPESP. Taphonomy is the study of how organisms decay and become fossilized or preserved in the paleontological record.
The principal investigator for this project was Fresia Ricardi-Branco, the penultimate author of the article and a professor at IG-UNICAMP.
“This was one of the first dinosaurs to walk the Earth, in the Triassic period,” she said. “The air sac adaptation enabled it to grow and withstand the climate in this period and later, in the Jurassic and Cretaceous. Air sacs gave dinosaurs an evolutionary advantage over other groups, such as mammals, and they were able to diversify faster.”
In a previous study, the group showed that the earliest fossils found so far did not have air sacs, taking their absence as a sign that this trait evolved at least three times independently.
M. itaquii was a biped, a sauropodomorph, and an ancestor of the giant quadrupeds with a small head, and a neck at least as long as the trunk.
Nonlinear evolution
Until air sacs were discovered in M. itaquii, these vertebral cavities were known to consist of either camerate or camellate tissue, the former referring to hollow spaces observed by microtomography, and the latter to spongy bone. According to the authors, in this case, they found “internal pneumatic chambers”, which are “neither camerate nor camellate, but a new type of tissue with an intermediate texture”. They propose to call the new structures “protocamerate”, as they “are not large enough to be considered camerae, but also present a camellate array internally”.
“The most widely held hypothesis until now was that the air sacs began as camerae and evolved into camellae. Our proposal, based on what we observed in this specimen, is that this other form existed first of all,” Aureliano said.
The vertebrae in which the air sacs were found also changed what was known about the evolution of these structures. Based on the fossils analyzed previously, other research groups proposed that air sacs first appeared in the abdominal region and did not appear in the cervical region until the early Jurassic (190 million years ago), a long time after the period in which M. itaquii was alive. Here, however, the authors found clear evidence of air sacs in the cervical and dorsal regions, with no sign of the structures in the abdominal region.
“It’s as if evolution had conducted different experiments until it arrived at the definitive system, in which air sacs run from the cervical region to the tail. It wasn’t a linear process,” Aureliano said.
Reference: “The origin of an invasive air sac system in sauropodomorph dinosaurs” by Tito Aureliano, Aline M. Ghilardi, Rodrigo T. Müller, Leonardo Kerber, Marcelo A. Fernandes, Fresia Ricardi-Branco and Mathew J. Wedel, 27 March 2023, The Anatomical Record.
DOI: 10.1002/ar.25209
Source: Report by SÃO PAULO RESEARCH FOUNDATION in scitechdaily.com
@WFS,World Fossil Society, Athira, Riffin T Sajeev, Russel T Sajeev













 November 3rd, 2023
November 3rd, 2023  Riffin
Riffin