Background
Living birds possess a unique heterogeneous pulmonary system composed of a rigid, dorsally-anchored lung and several compliant air sacs that operate as bellows, driving inspired air through the lung. Evidence from the fossil record for the origin and evolution of this system is extremely limited, because lungs do not fossilize and because the bellow-like air sacs in living birds only rarely penetrate (pneumatize) skeletal bone and thus leave a record of their presence.
Methodology/Principal Findings
We describe a new predatory dinosaur from Upper Cretaceous rocks in Argentina, Aerosteon riocoloradensis gen. et sp. nov., that exhibits extreme pneumatization of skeletal bone, including pneumatic hollowing of the furcula and ilium. In living birds, these two bones are pneumatized by diverticulae of air sacs (clavicular, abdominal) that are involved in pulmonary ventilation. We also describe several pneumatized gastralia (“stomach ribs”), which suggest that diverticulae of the air sac system were present in surface tissues of the thorax.
Conclusions/Significance
We present a four-phase model for the evolution of avian air sacs and costosternal-driven lung ventilation based on the known fossil record of theropod dinosaurs and osteological correlates in extant birds:
(1) Phase I—Elaboration of paraxial cervical air sacs in basal theropods no later than the earliest Late Triassic.
(2) Phase II—Differentiation of avian ventilatory air sacs, including both cranial (clavicular air sac) and caudal (abdominal air sac) divisions, in basal tetanurans during the Jurassic. A heterogeneous respiratory tract with compliant air sacs, in turn, suggests the presence of rigid, dorsally attached lungs with flow-through ventilation.
(3) Phase III—Evolution of a primitive costosternal pump in maniraptoriform theropods before the close of the Jurassic.
(4) Phase IV—Evolution of an advanced costosternal pump in maniraptoran theropods before the close of the Jurassic.
In addition, we conclude:
(5) The advent of avian unidirectional lung ventilation is not possible to pinpoint, as osteological correlates have yet to be identified for uni- or bidirectional lung ventilation.
(6) The origin and evolution of avian air sacs may have been driven by one or more of the following three factors: flow-through lung ventilation, locomotory balance, and/or thermal regulation.
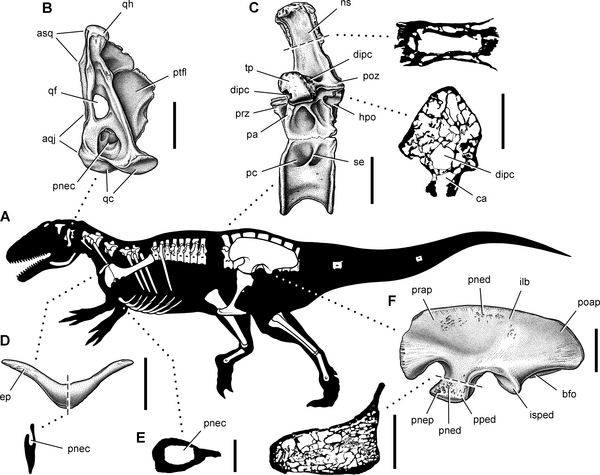
Summary of pneumatic features of the theropod Aerosteon riocoloradensis.
(A)-Silhouette reconstruction in left lateral view showing preserved bones of the holotype and referred specimens (MCNA-PV-3137-3139); body length approximately 9-10 m. (B)-Left quadrate in posterior view. (C)-Dorsal 14 in left lateral view with enlarged cross-sections of the neural spine and transverse process. (D)-Furcula in anterior view with sagittal cross-section. (E)-Cross-section of medial gastral element from the anterior end of the cuirass showing pneumatocoel. (F)-Left ilium in lateral view with enlarged cross-section of pubic peduncle. Scale bars equal 5 cm in B, 10 cm (3 cm for cross-sections) in C, 10 cm (same for cross-section) in D, 2 cm in E, and 20 cm (6 cm for cross-section) in F. Abbreviations: aqj, articular surface for the quadratojugal; asq, articular surface for the squamosal; bfo, brevis fossa; ca, canal; dipc, diapophyseal canal; ep, epicleideum; hpo, hyposphene; ilb, iliac blade; isped, ischial peduncle; ns, neural spine; pa, parapophysis; pc, pleurocoel; pnec, pneumatocoel; pned, pneumatic depression; pnep, pneumatopore; poap, postacetabular process; poz, postzygapophysis; pped, pubic peduncle; prap, preacetabular process; prz, prezygapophysis; ptfl, pterygoid flange; qc, quadrate condyles; qf, quadrate foramen; qh, quadrate head; se, septum; tp, transverse process.
doi:10.1371/journal.pone.0003303.g016
Citation: Sereno PC, Martinez RN, Wilson JA, Varricchio DJ, Alcober OA, et al. (2008) Evidence for Avian Intrathoracic Air Sacs in a New Predatory Dinosaur from Argentina. PLoS ONE 3(9): e3303. doi:10.1371/journal.pone.0003303
Editor: Tom Kemp, University of Oxford, United Kingdom



 July 22nd, 2013
July 22nd, 2013  Riffin
Riffin 
 Posted in
Posted in  Tags:
Tags: 