@WFS,World Fossil Society,Riffin T Sajeev,Russel T Sajeev
Morphological characteristics of preparator air-scribe marks: Implications for taphonomic research
Taphonomic analyses of bone-surface modifications can provide key insights into past biotic involvement with animal remains, as well as elucidate the context(s) of other biostratinomic (pre-burial) processes, diagenesis, excavation, preparation and storage. Such analyses, however, first require researchers to rigorously disambiguate between continuums of damage morphologies prior to attributing individual marks to specific actors and effectors (e.g., carnivore tooth, stone tool cutting edge, etc.). To date, a number of bone-modifying agents have been identified, and criteria for identifying their traces have been published. Relatively little research, however, has focused on bone-surface modifications imparted during specimen preparation. Herein we report that air scribes, small pneumatic tools commonly used for preparation in museum contexts, can generate unintentional marks that may mimic surficial modification caused by carnivores. To aid investigators in assessing the hypothesis that a mark in question is derived from air-scribe preparation activities, we provide high-resolution, detailed morphological information imaged with scanning electron microscopy (SEM). The main diagnostic characteristic of air-scribe damage is the occurrence of sequential, variously spaced, sub-millimeter scallop-like stepped bone removals. This morphology can resemble damage imparted by carnivore teeth. In contrast to marks produced by trampling, stone tools and carnivores, however, no continuous internal features, such as linear microstriations, were observed within grooves produced by the air scribe. Thus, the presence of such features can be used to disprove an air-scribe origin. A culmination of the morphological criteria presented herein, cross-cutting relationships with other surficial features (e.g., diagenetic discoloration, weathering textures), the position of occurrence, and an overall contextual framework for the assemblage is suggested for accurate identification of such traces. The ability to recognize or disprove air-scribe damage will allow researchers to confidently proceed with interpreting past biological and sedimentological interactions with animal remains.
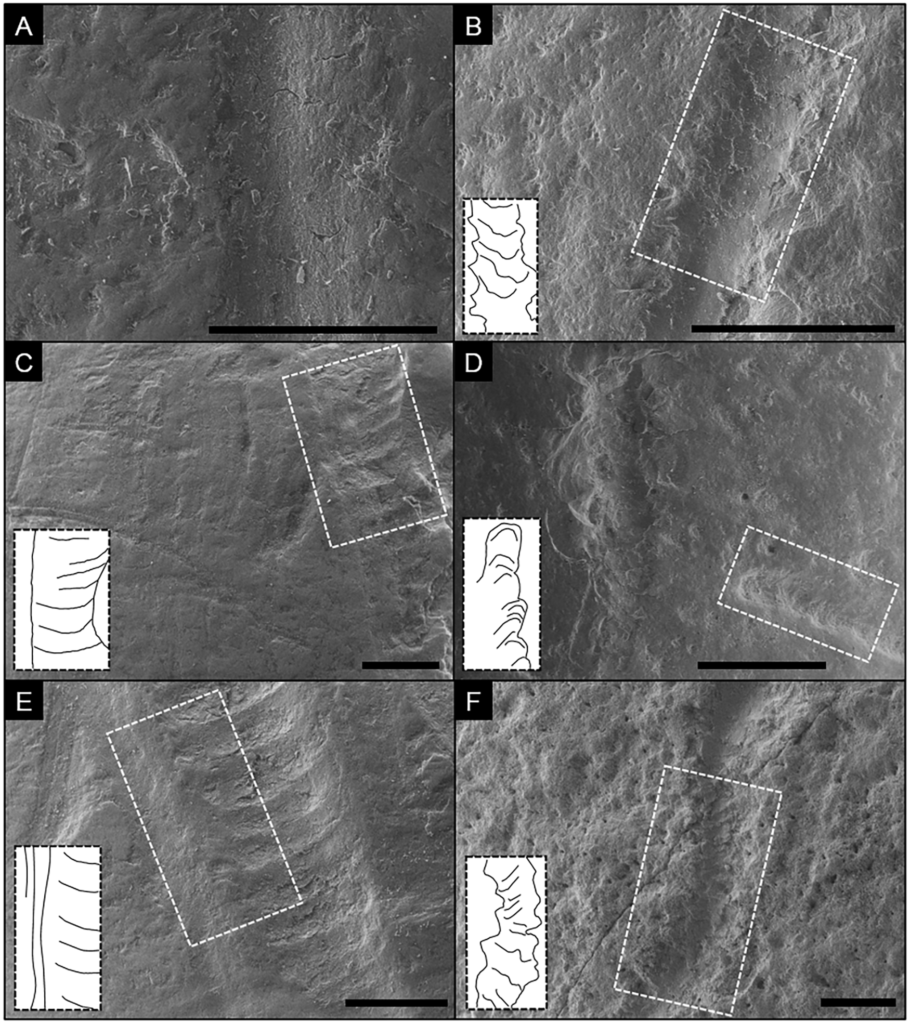
Comparison of carnivore-induced tooth scores (left column) and air-scribe-induced grooves (right column) under SEM.
Insets are interpretive drawings of highlighted areas. A) Segment of a smooth tooth score with relatively smooth margins and faint, internal grooves exhibiting continuity on a bovid long bone; scale bar = 0.5 mm. B) Segment of a transition within an air-scribe groove from a chatter-like morphology (upper half) into a smooth-based groove on a fossilized bone fragment; scale bar = 1.0 mm; Notice the rougher margins in comparison to A. C) Shallow tooth score with chatter marks, a smooth left margin, and an irregular right margin on a bovid long bone; scale bar = 1.0 mm. D) shallow (lower left) and deep (top to bottom) air-scribe grooves on a fossilized bone fragment; scale bar = 1.0 mm. Notice the similarities between the chatter morphology of the lower-left mark and the scores depicted in C and E. Also note the relatively rougher margins. E) Tooth score with pronounced chatter markings and a long-axis-parallel, smooth left shoulder with through-going continuity on a bovid long bone; scale bar = 1.0 mm. F) sinuous air-scribe groove with faint chatter marks, rough margins, and an absence of through-going continuity on a fragment of a chelonian carapace; scale bar = 1.0 mm.
Citation: Wiest LA, Ferraro JV, Binetti KM, Forman SL, Esker DA, Kibunjia M, et al. (2018) Morphological characteristics of preparator air-scribe marks: Implications for taphonomic research. PLoS ONE 13(12): e0209330. https://doi.org/10.1371/journal.pone.0209330
Editor: Cyril Charles, Ecole Normale Supérieure de Lyon, FRANCE
@WFS,World Fossil Society,Riffin T Sajeev,Russel T Sajeev













 November 3rd, 2019
November 3rd, 2019  Riffin
Riffin 



![Distribution of tetrapod Konservat-Lagerstätten through time and space (n = 143). (a) Numbers of Lagerstätten found in fluvial (light green), lacustrine (medium green), near-shore marine (blue) and other terrestrial depositional environments (dark green) are shown on the y-axis by period as well as epoch (for the Mesozoic and Cenozoic). For all statistical analyses, we used 10 My time bins after assessing the influence of time bin size on our statistical results (see electronic supplementary material, figure S7). (b) Global distribution of Lagerstätten found in different depositional environments. Pies show relative numbers in different depositional environments (colours of pie) and absolute number per country (size of pie). (c) Earliest skin impressions in Saurerpeton [49]. (d) Earliest putative filaments in Eudimorphodon rosenfeldi [50]. (e) Scales in the ornithischian dinosaur Kulindadromeus zabaikalicus [24]. (f) Earliest known feathers in Anchiornis huxleyi [51]. (g) Earliest preserved hair in Rugosodon eurasiaticus [52]. Image credits: (c) Smokeybjb (CC BY-SA 3.0), (d) Tommy from Arad (CC BY 2.0), (e) Tomopteryx (CC BY-SA 4.0), (f) Kumiko (CC BY-SA 2.0), (g) Zhe-Xi Luo (University of Chicago). (Online version in colour.)](http://www.worldfossilsociety.org/wp-content/uploads/2019/09/190220161034-ancient-finds-exlarge-169-2-1024x745.jpg)


